

The study, “A Multi Center, Prospective, Randomized, Controlled Trial, Comparing the Safety and Efficacy of ActiGraftPRO to Standard of Care in Patients with Chronic Neuropathic Diabetic Foot Ulcers,” (NCT04185558) included 119 patients at 16 sites across the world.
Results were recently published in the Journal of Wound Care.
The ActiGraftPRO clinical trial was conducted with utmost rigor, adhering to the highest standards of trial design and execution.
An independent remote blinded assessor reviewed all images for healing and measurement accuracy by tracing validation.
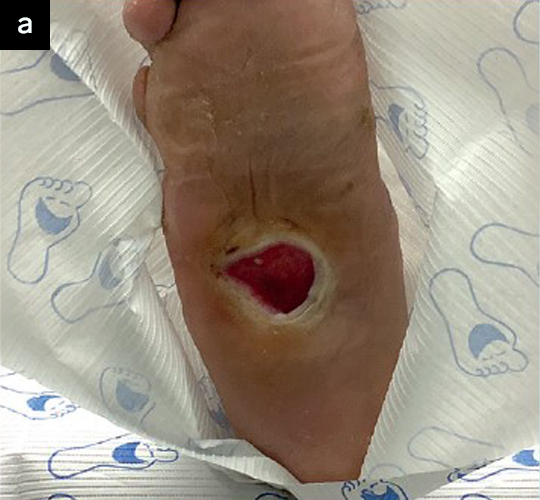
ActiGraftPRO is an autologous blood clot, point wound management solution created from your patient’s whole blood. Once applied, the blood clot serves as a protective wound covering and optimizes the body’s own healing potential.
Complete Wound Closure (ITT) Population


Complete Wound Closure (PP) Population







Fill out the form on the right.
Reference
1. Snyder R, Nouvong A, Ulloa J, et al. Efficacy and safety of autologous whole blood clot in diabetic foot ulcers: a randomised controlled trial. Journal of Wound Care. 2024; Published Online: 30 Aug 2024. https://doi.org/10.12968/jowc.2024.0195
Solutions
Copyright RedDress © 2024